- Visibility 804 Views
- Downloads 96 Downloads
- Permissions
- DOI 10.18231/j.ijpp.2024.032
-
CrossMark
- Citation
The natural flavone quercetin: An extensive analysis of its pharmacological mechanisms and medicinal prospects
Abstract
Natural flavonoid quercetin is widely distributed in fruits, vegetables, and medicinal plants. It has attracted much interest due to its wide range of biological advantages and potential as a treatment for several illnesses. To better understand the mechanisms behind the therapeutic of effects quercetin in cancer, obesity, diabetes, and osteoporosis, this review attempts to present a thorough overview of these mechanisms. By promoting osteoblast differentiation, reducing osteoclast activity, and increasing bone mineral density, quercetin shows promise as a protective agent against bone loss in osteoporosis. Its anti-inflammatory properties also help to reduce the risk of fracture and bone resorption linked to osteoporosis. Through its targeting of various signalling pathways involved in cell proliferation, apoptosis, angiogenesis, and metastasis, quercetin shows promising anti-cancer properties in cancer. Moreover, its effectiveness in reducing the progression of cancer is attributed to its capacity to regulate inflammation and oxidative stress. Through its effects on adipocyte differentiation, glucose uptake, insulin signalling, and lipid metabolism, quercetin shows anti-obesity and anti-diabetic effects in obesity and diabetes. Furthermore, the antioxidant and anti-inflammatory qualities of quercetin are essential in reducing the negative effects of obesity on insulin resistance and the complications associated with diabetes. In summary, this review offers valuable implications for the development of quercetin-based therapeutics and nutraceuticals for disease management by shedding light on the complex mechanisms through which quercetin exerts its biological benefits in different disease contexts.
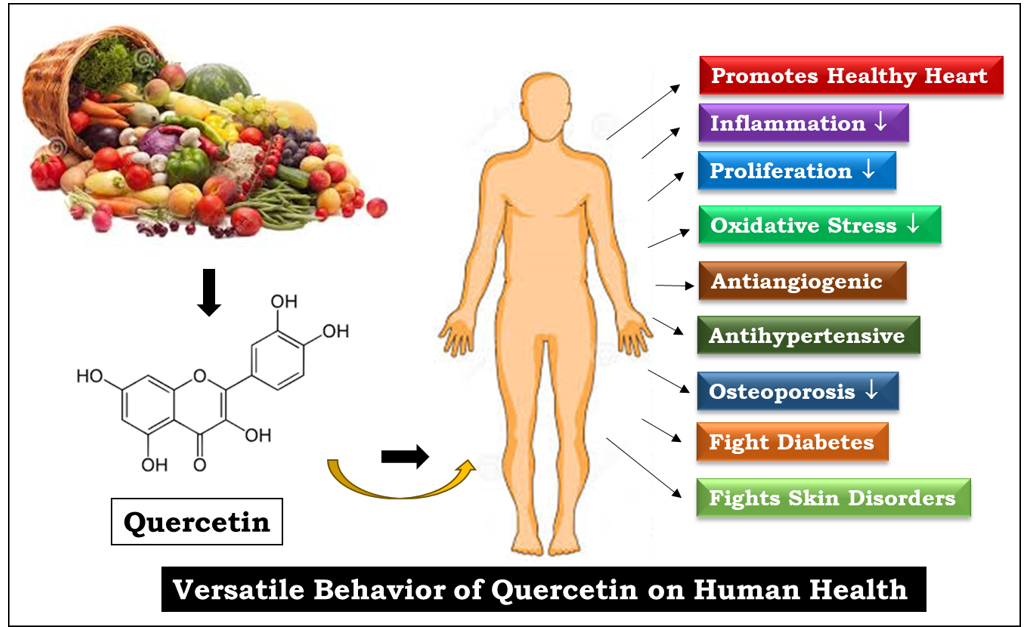
Introduction
Quercetin is a flavonoid polyphenol that is considered an important phytochemical. Strong antioxidant flavonoid quercetin, also known as flavanol, is a plant pigment found mostly in onions, grapes, berries, cherries, broccoli, and citrus fruits. This versatile antioxidant has demonstrated defense capabilities against tissue deterioration caused by various drug toxicity levels. [1]
Using plants and their phytoconstituents instead of pharmaceuticals has several advantages. It has been established that plant extracts and phytoconstituents have biological effects, such as the capacity to scavenge free radicals, lessen inflammatory disorders, and treat hyperlipidemia and diabetes. Most metabolic diseases hurt the quality of life and are primarily caused by free radicals. In the balanced environment that nature provides a balanced system, one can live a pleasant and healthy life. In recent decades, there has been an increased focus on finding compounds that have antioxidant properties. [2]
For the past 30 years, researchers have focused a great deal of attention on quercetin, a special kind of bioflavonoid. Nobel laureate Albert Szent Gyorgyi made the 1930 discovery of bioflavonoids. [3] Flavonoids are a class of naturally occurring substances that vary in their phenolic structure. They can be found in tea, wine, grains, fruits, vegetables, bark, roots, and stems. Before flavonoids were identified as beneficial compounds, these natural products were well-known for their positive health effects. More than 4000 different types of flavonoids are known to exist, and many of them give flowers, fruits, and leaves their lovely colors. [4]
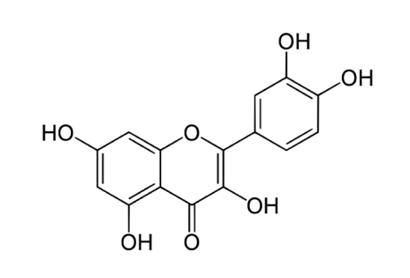
The Latin word "Quercetum," which means "Oak Forest," is the source of the name quercetin (3,3',4',5,7-pentahydroxyflavone) ([Figure 2]), which is a member of the flavonol class that the human body is unable to produce. It has a yellow hue, dissolves poorly in hot water, dissolves well in lipids and alcohol, and is insoluble in cold water. [5]
Quercetin has various pharmacological characteristics, including anti-inflammatory, anti-cancer, antiviral, antibacterial, neurologically protective, and anti-obesity effects. [1] Recently, quercetin was granted the GRAS (Generally Recognized as Safe) designation by the US Food and Drug Administration (FDA). [6] This review aims to summarize the pharmacological mechanism of the bioactive flavonoid quercetin.
Discussion
Quercetin role in osteoporosis (Op)
Patients with OP, a common bone disorder, are more likely to fracture because of their weakened bone architecture and decreased bone mineral density. Pharmacotherapy is the main method used to treat OP, and recently, interest in the phytochemical compound quercetin has grown. Through anti-inflammatory and antioxidative mechanisms, this naturally occurring flavonoid supports bone health and metabolic balance while regulating bone marrow mesenchymal stem cells, osteoblasts, and osteoclasts.[7]
The process through which quercetin facilitates osteoblast-mediated bone growth
Four phases of bone formation are experienced by osteoblasts (OBs): proliferation, extracellular matrix mineralization, extracellular matrix maturation, and apoptosis. [8] Throughout different developmental stages, they are influenced by an array of transcription factors and signaling pathways that ultimately culminate in the completion of normal bone formation. [9] The mechanism through which quercetin promotes osteoblast-mediated bone formation ([Figure 3]).
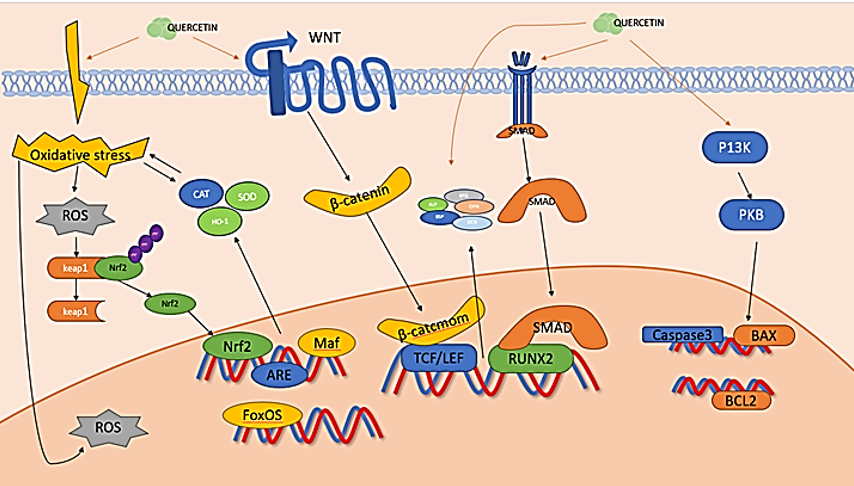
OB-specific Mesenchymal stem cells (MSCs) must differentiate into OBs for functional OBs to arise, and this requires the transcription factors runt-associated transcription factor 2/corebinding factor alpha 1 and special protein 7 transcription factor (osterix). [10] Osterix, which is produced only in bone tissue cells and functions as a downstream gene of RUNX2 to play an osteogenic role, is necessary for the differentiation of OBs and the production of new bone. [11] RUNX2 and SP7 mRNA expression levels are demonstrated to be significantly elevated in mouse bone marrow mesenchymal stem cells (mBMSCs) upon administration of 5 μM Quercetin. The conventional Wnt signaling pathway significantly regulates bone resorption and formation. [12] A secreted glycoprotein called Dickkopf-1 binds to LRP5/6 receptors with Wnt proteins competitively, inhibiting the Wnt/β-catenin signaling pathway and leading to bone resorption. quercetin stimulates the osteogenic differentiation of MC3T3-E1 cells by upregulating the levels of β-catenin protein and initiating the Wnt/β-catenin pathway.[13] Quercetin promotes the growth and osteogenic differentiation of BMSCs by activating the downstream Wnt/β-catenin pathway by targeting the H19/miR-625-5p axis. However, high concentrations of Quercetin (10 μmol/L) inhibit the Wnt/β-catenin pathway. [14] Quercetin (2 or 5 μM) significantly increases the mRNA levels of ALP, RUNX2, BMP2, and other OB marker genes, and enhances the relative alkaline phosphatase (ALP) activity and matrix mineralization of third-generation mBMSCs, thereby promoting their osteogenic development. [15] Quercetin also enhances the BMP signaling pathway's activation via the endoplasmic reticulum, upregulates the expression of genes that are downstream of it, including OSX, RUNX2, and OPN, and encourages BMSC proliferation and osteogenic differentiation. [16] Quercetin may suppress the production of ROS and FAC-induced apoptosis, upregulate the expression of Bcl-2, and downregulate the expression of caspase-3 and Bax. [17] Quercetin can reduce the excessive production of intracellular ROS brought on by sodium nitroprusside and restore the potential of the mitochondrial membrane, which will lessen chondrocyte apoptosis. This is achieved by altering the Bcl-2/Bax-caspase-3 signaling pathway. [18] Cellular defense mechanisms are strengthened against oxidative damage by upregulating Nrf2 and HO-1 expression during oxidative stress. [19] Quercetin has a dose-dependent effect on BMSCs' HO-1 mRNA expression. [20] It significantly increases Nrf2 nuclear translocation in FAC-induced MC3T3-E1 cells and decreases FAC-induced oxidative stress injury by inducing the Nrf2/HO1 signaling pathway. [21] In addition to increasing the number of mineralized nodules and mineralized matrix accumulation in BMSCs, quercetin treatment significantly increases the expression of OCN and OPN mRNA in BMSCs. Additionally, it encourages BMSCs to develop into osteogenic cells. Pretreatment with Quercetin significantly restores bone mineralization and OCN mRNA and protein expression levels in lipopolysaccharide (LPS)-inhibited MC3T3-E1 cells in a dose-dependent manner. [22]
OC-mediated bone resorption inhibition by Quercetin
Osteoclasts (OCs) are multinucleated giant cells with the ability to resorb bone that is created when bone marrow monocyte precursors fuse.[23] They promote bone resorption and remodeling by secreting acids and enzymes that degrade the bone matrix. When OCs are overactive or excessive, the balance between OBs and OCs is disrupted, which can lead to excessive bone resorption and OP. Transcription factors regulate the activity and differentiation of OCs. [24] The nuclear factor of activated T cell 1 is the primary regulator of OC differentiation (NFATc1). [25] It controls OC activity and OC-specific genes such as TRAP, Ctsk, calcitonin receptor, and OC-associated receptor by cooperatively activating nuclear factor kappa B (NF-κB), c-Fos, c-jun, and microphthalmia-associated transcription factor (Mitf). Quercetin therapy inhibits the NFATc1 gene and protein expression via the TRAF6/c-fos/NFATc1 signaling pathway, preventing RAW264.7 cells from differentiating into OCs. By inhibiting the expression of NFATc1 induced by RANKL, que-3-O-β-D-glucoside prevents both bone loss and OC differentiation. [26]
The OPG/RANKL/RANK signaling pathway is a crucial signaling axis during bone remodeling ([Figure 4]). In the presence of M-CSF, RANK binds to the C-terminus of RANKL, activating transcription and expression of downstream OC-specific genes and attracting factors associated with the tumor necrosis factor receptor.[27] Upon implanting OB-OC-endothelial cells in three cultures on Que-containing hydroxyapatite, the trend of OPG/RANKL levels correlated with the reduction in histone K levels, suggesting that Que inhibits the viability of OC. [28]
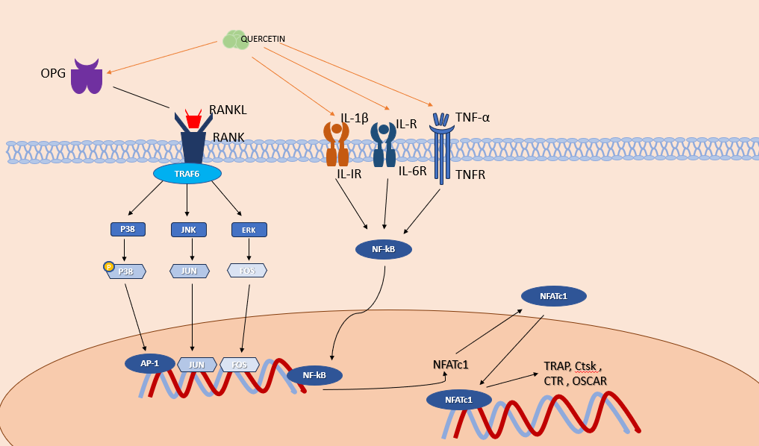
The main participants in the growth of OC precursors and OC apoptosis, respectively, among the OC precursors are two ERK forms (ERK1/2) and three JNK isoforms (JNK1/2/3). [29] Qué-3-O-β-D-glucuronide significantly lowers JNK and ERK activation in LPS-stimulated RAW264.7 macrophages. [30] Due to its concentration-dependent anti-inflammatory properties, it inhibits the release of PGE and plasmatic NO, as well as the production of COX-2 and iNOS. quercetin acts through the JNK-c-Jun/AP-1 and ERK-c-Fos/AP-1 pathways to prevent apoptosis. [31] The dynamic regulation of both osteoclastogenic and anti-osteoclastogenic cytokines is necessary for maintaining bone homeostasis. Tumor necrosis factor-α (TNF-α) drives OC development by upregulating RANK pro-inflammatory target genes, promoting NF-κB nuclear translocation, disrupting the balance of the RANK-RANKL bio-axis, and increasing OC activity. [32] TNFα and IL-6 accumulation in LPS-induced murine RAW264.7 macrophages is significantly reduced by Que (2 or 5 μM). [33] In macrophages and microglia, que suppresses M1 polarization and significantly reduces the expression levels of M1 markers such as IL-6, TNF-α, and IL-1β. Quercetin inhibits OC activation, significantly reduces TNF-α and IL-1β levels, and minimizes bone loss.[34]
Quercetin Role nn Cvs Conditions
Acute myocardial infarction
Acute myocardial infarction (AMI), which causes coronary artery blockage, suppression, and interruption of the blood supply to the heart tissues, is the fundamental cause of coronary artery disease. Quercetin is a flavonoid compound with several advantageous properties. A few of these include reducing blood pressure and scavenging reactive oxygen species (ROS), shielding heart tissues from ischemia and ischemia-reperfusion damage, adjusting immune system activity, and encouraging antioxidant activities.[35] Strong antioxidative stress properties of Quercetin can be utilized to stop AMI. Quercetin inhibits each type of cardiac dysfunction caused by chronic unpredictable stress (CUS). Quercetin inhibits the CUS-ST segment elevation in rats.[36] Quercetin stops the reduction of the GPx antioxidant enzyme when it is induced by CUS. When rats are administered MI, Quercetin keeps their heart muscle cells from dying. Before ischemia, Quercetin pretreatment protects the heart's myocardial tissue from oxidation and inflammation.[37] Quercetin postconditioning prevents ischemia/reperfusion via the PI3K/Akt signaling pathway, which reduces apoptosis, though the exact mechanism by which it does so is still unknown. [38] Quercetin post-conditioning dramatically decreased the amount of the infarct and myocardial cell death after myocardial infarction. Quercetin has anti-ischemic effects. It not only protects the heart from MI damage but also reverses its harmful effects, such as apoptosis, structural changes, and matrix metalloproteinase 2 activation. Quercetin or its supplements are therefore present to prevent myocardial infarction.[39]
Hypertension
QU showed its antihypertensive properties by modifying the kidneys' arachidonic acid (AA). The main metabolites of AA that regulate arterial blood pressure are 20-HETE and EETs. [40] QU is one of the most potent radical oxygen and nitrogen species scavengers. QU significantly reduces MDA, AOPP, and H2O2 levels in the kidney while increasing CAT, SOD, and GSH levels. All of these signs point to quercetin's potential as an antioxidant. NO production improves when inflammation is reduced. NO, an innate vasodilator, can relax blood vessels and smooth muscle contraction.[41] QU dramatically increases PGI2 and COX2 levels, which lower blood pressure. ROS affects blood pressure by lowering the production of NO. It is more common to impact the later stages of severe hypertension than the earlier ones.[42] QU improves endothelial cell function by scavenging reactive oxygen species. QU inhibits the ACE function in a dose-dependent manner. QU primarily reduces blood pressure by destroying bradykinin and blocking angiotensin II. The principal mechanism by which quercetin lowers blood pressure is through its inhibition of renin-angiotensin-aldosterone (RAAS) and contraction of the VSMC.[43] Conversely, in rat models of metabolic disorders, diabetes, and other conditions, quercetin can reduce hypertension. QU has been reported to possess properties that inhibit the Ca2+ channel. Furthermore, by opening Ca2+-activated K+ channels, which in particular cause cells to become hyperpolarized, QU lessens endothelial dysfunction. Increased NO production through membrane hyperpolarization-induced capacitive Ca2+, which is dependent on Ca2+-activated K+ channels, is the cause of this effect.[44]
Cardiac arrhythmia
One of the most common cardiovascular conditions in the world, cardiac arrhythmia can be deadly in extreme circumstances and can arise on its own or as a result of other conditions. QU controls autophagic response and dramatically reduces inflammatory responses, cardiac fibrosis, mitochondrial oxidative stress, and apoptosis.[45] Additionally, QU opens the vital mitoKATP channels for heart health. A mitoKATP is one kind of potassium channel that regulates cell activity and protects heart cells from damage caused by free radicals.[46] QU may have antiarrhythmic effects in this method. QU can treat ventricular arrhythmias in rats.[47] Similarly, QU and its analogs could inhibit ATXII-induced late sodium currents in rat ventricular myocytes and improve calcium handling and cardiomyocyte contractility to produce antiarrhythmic effects. QU, however, may have cardioprotective and antiarrhythmic effects because it is a cardiac voltage-gated inhibitor.[48]
Atherosclerosis
Endothelial cells are the first to defend hemostasis during cardiovascular events. The endothelium ages, which promotes atherosclerosis.[49] Quercetin protects vessels by increasing blood flow within arteries. In addition, quercetin prevents lipid aggregation and decreases blood levels of LDL, TNF-a, IL-1B, IL-18, and IL-6. Quercetin's capacity to inhibit ROS may contribute to the prevention of atherosclerotic plaques.[50] By giving 20 mg/kg/d Quercetin for eight weeks, lipid deficiencies in vascular intima can be effectively alleviated and treatments for atherosclerosis can be developed.[51] One of the recently discovered compounds from quercetin, quercetin 7-O sialic acid, has stronger antiatherosclerosis effects than quercetin alone when combined with N acetylneuraminic acid and quercetin. NADPH oxidase is necessary for the development of atherosclerotic lesions in rats, according to a report from a different study. Based on this data, it was concluded that quercetin controls NADH oxidase activity. Additionally, quercetin can regulate ABCA1 levels.[52] Quercetin functions by causing cholesterol to exit the body more quickly and by stopping macrophages from producing foam cells. Quercetin increases the number of LXRs because it is a receptor involved in cholesterol metabolism that can coordinate lipid metabolism. There is a novel signaling pathway linked to the suppression of inflammation that lies between several molecular pathways that are responsible for the antiatherosclerosis properties of quercetin. [53] It has been reported that the administration of 100 mg/kg quercetin inhibited the NLRP3 inflammatory activities of macrophages for 16 weeks. Additionally, QU inhibits the synthesis of galectin-3, a material that promotes the formation of atherosclerotic plaques.[54] Quercetin has been associated with a decrease in atherosclerosis through the regulation of autophagy. The vast majority of cells linked to atherosclerosis are T lymphocytes and macrophages. When quercetin and docosahexaenoic acid are combined, NF-KB expressions are suppressed. However, quercetin alone can reduce NF-KB translocation, suggesting that it may be a potential preventive measure for atherosclerosis.[55]
Myocarditis
Myocarditis is the term for heart inflammation. It is connected to cell necrosis and cardiac degeneration. Autoinflammation is a major contributing factor in the etiology of myocarditis, despite the lack of a specific medication for the condition. The induction of experimental autoimmune myocarditis (EAM) is associated with the secretion of cytokines by macrophages and T cells.[56] Moreover, QU affects activated macrophages' nitric oxide production. It has been demonstrated that QU is helpful in the treatment of autoimmune-related diseases. The severity of autoimmune myocarditis was significantly reduced after three weeks of QU administration. QU lessens the degree of inflammation by upregulating IL-10 and suppressing proinflammatory cytokines like TNF-α and IL-17. [57]
Quercetin Role in Inflammation
In vitro synthesis of cyclooxygenase (COX) and lipoxygenase (LOX), which are normally stimulated by inflammation, has been demonstrated to be inhibited by quercetin.[58] Furthermore, studies conducted in vivo have validated the anti-inflammatory properties. One example of quercetin's inhibitory effects is the noticeable reduction of proinflammatory cytokines in cultured fibroblasts.[59] 10𝜇M quercetin downregulated the synthesis of COX-2, NO, and nuclear factor kappa B (NF-𝜇B). NO, and TNF-𝜇 were lowered by quercetin at concentrations ranging from 10 to 25𝜇M. At 50 and 100 𝜇M, quercetin has been demonstrated to have extra advantages, such as reducing the production of TNF-𝜇and IL-6 in LPS-stimulated RAW 264.7 macrophages.[60], [61] Nonetheless, it was most successful in preventing TNF-𝜇 release in macrophages at concentrations between 25 and 50 𝜇M. Finally, at low doses, quercetin (less than 50𝜇M) dramatically increased the anti-inflammatory cytokine IL-10.[62] Similarly, 25𝜇M quercetin inhibited LPS-induced production of TNF, IL-1𝜇, IL-6, and IFN-𝜇 in human whole blood. Furthermore, it has been demonstrated that quercetin is an effective pretreatment for apoptotic cell death. Moreover, quercetin would stop stress-activated protein kinases (JNK/SAPK) and p38 MAPK from becoming activated, which would stop the growth-inhibiting effect on cells. Quercetin appears to have potential as a weapon against inflammatory diseases based on several data points. Moreover, it might be advantageous for cells involved in allergic inflammation.[63]
Inflammation plays a key role in how CAD develops. Increased levels of proinflammatory cytokines stimulate immune-competent and endothelial cells. NF-kB, TNF-α, and IL-1β activations are involved in the development of CAD. Quercetin is associated with a decrease in IL-1β and NF-kB gene expression. It not only inhibits the activation of inflammatory genes but also activates the protein sirtuin 1 (SIRT1). [64] QU prevented endothelial cell damage caused by the SIRT1 protein. QU also prevents lipid peroxidation damage and has potential in the treatment of CAD. Following a two-month regimen of QU 120 mg daily, 85 patients with CAD demonstrated positive characteristics.[65] QU eliminates free radicals and reduces the body's oxidation of low-density lipoproteins. QU therapy, which protects cells from oxidative damage, is administered for two months to eighty patients with hyperlipidemia.[66] In individuals with CAD, QU at a dosage of 3 grams per day for two months lowers necrosis factor and endothelial cell degeneration. QU is suggested as a potential Angioprotector agent since it increases the statin drug's efficacy.[67]
Quercetin role in fat
Research indicates that QU downregulated both apoptosis and adipogenesis by inhibiting the activity of enzymes associated with adipogenesis. Meanwhile, there was an increase in MAPK and its substrate, acetyl-CoA carboxylase (ACC).[68] Apart from triggering programmed cell death, JNK and ERK phosphorylation were also reduced. It is suggested that by triggering the MAPK signaling pathway, quercetin prevents the process of adipogenesis. At the same time, quercetin blocked the important JNK and ERK pathways, which led to the apoptosis of mature adipocytes. According to some researchers, quercetin regulates lipid metabolism and the expression of genes in the liver.[69] Studies have shown that quercetin shields C57B1/6 mice against obesity brought on by a high-fat diet (HFD) by potentially regulating lipogenesis. Significant reductions in the amount of obesity resulting from the diet were observed in mice supplemented with quercetin rather than following the HFD without the supplement.[70] The weight of the mice's liver, total white adipose tissue, and body all decreased in the supplement-fed mice. It seems that quercetin's gene profiles linked to lipid metabolism have altered.[71]
Quercetin Role in Cancer
Anti-proliferation effect
A major factor in the prevention of cancer is the tumor suppressor protein p53, which regulates the cell cycle, apoptosis, and DNA repair.[72] Research indicates that quercetin either activates or stabilizes p53, causing hepatocellular carcinoma (HCC) cells to enter cell cycle arrest and eventually die.[73] QUs anti-proliferative effect was demonstrated in a different study where it decreased intracellular ROS in HCC cells without affecting p53 expression. Protein kinase C (PKC) and phosphatidyl 3-kinase (PI3K) both play a similar role in enhancing cell survival and proliferation. Through the reduction of PKC, PI3K, and cyclooxygenase (COX-2), quercetin enhanced the expression of p53 and BAX in hepatocellular carcinoma (HepG2) cell lines ([Figure 5]). [74] As a result, fewer cancer cells developed. It has been demonstrated that quercetin inhibits the growth of melanoma cells A375 by controlling the Wnt/b-catenin signaling pathway proteins, which include DVL2, b-catenin, cyclin D1, Cox2, and Axin2.[75] Research on quercetin's effects on the growth and cell cycle of human gastric cancer cells has revealed a connection between the supplement's suppression of the G1 phase of the cell cycle and the proliferation of gastric cancer.[76]
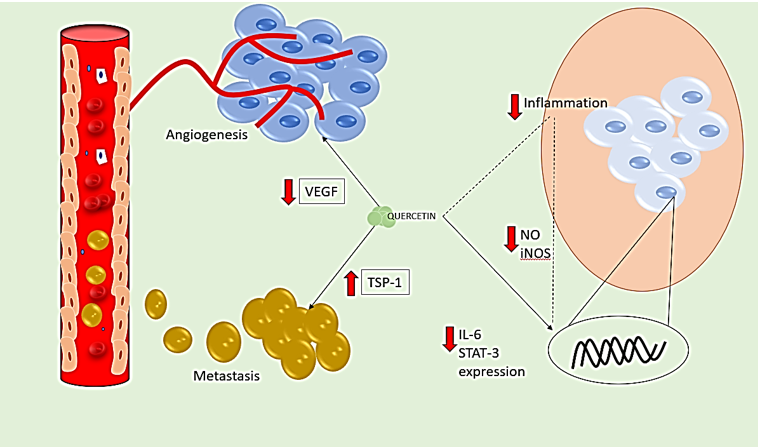
Anti-angiogenesis effect
Angiogenesis, the process that produces all capillaries, is regulated by several substances, including endostatin, adhesion molecules, and growth factors. An important factor in tumor angiogenesis is the interaction between tumor cells and endothelial cells. [77] VEGF is essential for the growth and survival of endothelial cells, as well as for promoting cell proliferation, raising vascular permeability, extravasating plasma fibrin, depositing cellulose, and stimulating tumor angiogenesis. [78] A diet high in flavonoids may reduce the risk of cancer, according to epidemiological research. Quercetin has shown anti-tumor effects by slowing the growth of blood vessels. [79]
Quercetin's Role in Skin Health
QU protects against UV-induced skin damage by suppressing metalloproteinase-1 (MMP-1), a protease enzyme that degrades collagen, as well as lowering AP-1 activity. It interrupts signal transduction pathways to boost NF-κB antagonist activity. It also inhibits cytokine-mediated inflammatory cascade activation and IL-1-stimulated human mast cell IL-6 production. [80] QU also suppresses nitric oxide synthesis and downregulation of nitric oxide synthase, as well as TNF-α expression and function, in mouse models. [81] In acute inflammation, QU has a dose-dependent effect on leukocyte recruitment, lipid peroxidation, chemokine and ROS levels, and antioxidant enzyme activity. It interferes with the balance and synthesis of pro-inflammatory cytokines like IL-17 and TNF-α and anti-inflammatory cytokines like IL-10. By reducing oxidative stress and NF-κB activity, it prevents chronic inflammation by reducing immune cell activation and accumulation on a global scale. [82] Furthermore, it has been shown to work in concert with vitamin C, as ascorbate recycles QU, making it more effective. Its capacity to mend wounds has also been the subject of numerous investigations. [83]
QU provides skin-soothing and regenerative properties that promote healing in damaged and aging skin by stabilizing moisture loss from UV and chemical exposure, promoting the regeneration of the skin barrier, and restoring normal hydration levels. [84] Its anti-oxidant properties increase the viability of cells and protect the skin from oxidative stress brought on by extended exposure. Its anti-MMP action is complemented by its senolytic properties and capacity to encourage the renewal of cellular morphology. Since some research suggests quercetin glycosides may have anti-melanogenesis effects, they may be used as a whitening treatment to treat dark, aging patches.[85]
Quercetin Role in Diabetes
Quercetin has anti-diabetic properties by promoting glucose absorption through an insulin-dependent MAPK pathway. In response to the mechanism's activation in skeletal muscles, the translocation of glucose transporter 4 (GLUT4) has been seen. In contrast, the liver uses MAPK primarily to downregulate the major gluconeogenesis enzymes, thereby inhibiting the production of sugar.[86] According to the homeostasis model, the quercetin-treated group's insulin did not rise or fall, and their glucose plasma levels were lower than those of the control group. Animals given 0.08% quercetin also exhibited improvements in liver enzyme activities vital to detoxification processes, reductions in plasma total cholesterol and triacylglycerols, and increases in plasma adiponectin and HDL-cholesterol. [87] By suppressing the overexpression of connective tissue growth factor (CTGF) and preventing the overexpression of TGF-θ1, quercetin has also been shown to improve renal function in diabetic nephropathic rats. End-stage renal disease and diabetic nephropathy are closely associated conditions. Studies reveal that TGF-μ1 and CTGF are essential for the pathophysiological mechanisms of DN. Quercetin's effects on TGF 1 and CTGF renal functions in diabetic Sprague-Dawley rats treated with streptozotocin (STZ) were studied.[88] The results showed that rats given quercetin had a lower kidney-to-body weight ratio. The expression of CTGF and TGF 1 is higher in kidney tissues. Those who took quercetin experienced a decrease in overexpression. Finally, it has been shown that quercetin creates an effective in vitro barrier against lens aldose reductase in addition to inhibiting polyol accumulation.[89] It has been shown that quercetin helps people with type 2 diabetic neuropathy feel less irritated, numb, and shocked. Furthermore, it has been shown that quercetin treatment improves several quality-of-life measures.[90]
Quercetin's Role in Neurodiverse
It is commonly accepted that oxidative stress is a major factor in a variety of neurodegenerative diseases, mediates the harmful effects of some neurotoxicants, and is a mechanism for degenerative processes associated with aging. Reactive oxygen species (ROS) cause oxidative stress and damage proteins, lipids, and DNA when they accumulate in cells due to excessive synthesis or insufficient neutralization. The mitochondria are one of the primary sources of ROS in cells; ROS produced there can also target complex I and other electron transport chain components, initiating a cycle that ultimately results in ATP depletion and cell death. [91] Quercetin furthermore inhibits neurodegeneration by controlling autophagy. It has been shown that quercetin reduces the damage that extreme hyperglycemia does to Schwann cell cells by inducing autophagy. Similarly, quercetin increases autophagy in C. elegans to counteract the neurotoxicity of amyloid beta.[92], [93]
Conclusion
The thorough analysis of quercetin concludes by highlighting its extraordinary potential to treat various health issues. Quercetin is a multifunctional agent that promotes overall well-being due to its ability to fight cancer, osteoporosis, and cardiovascular diseases. It also has promising anti-diabetic and anti-obesity properties. Our review underlines the significance of quercetin as a promising therapeutic avenue for various health conditions by elucidating its diverse mechanisms of action.
Conflict of Interest
There is no conflict of interest among the authors of any source including financial or non-financial.
Source of Funding
None.
Acknowledgement
The authors are thankful to Smt Kishortai Bhoyar College of Pharmacy, kamptee for providing the infrastructural facility to carry out the review work.
References
- David A, Arulmoli R, Parasuraman S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Phcog Rev. 2016;10(20):84-9. [Google Scholar]
- Tran N, Pham B, Le L. Bioactive Compounds in Anti-Diabetic Plants: From Herbal Medicine to Modern Drug Discovery. Biology. 2020;9(9). [Google Scholar]
- Lakhanpal P, Rai D. Quercetin: A Versatile Flavonoid. Internet J Med Update - ejournal. 2007;2(2):22`-37. [Google Scholar]
- Abarikwu S. Protective Effect of Quercetin on Atrazine-Induced Oxidative Stress in the Liver, Kidney, Brain, and Heart of Adult Wistar Rats. Toxicol Int. 2014;21(2):148-55. [Google Scholar]
- Maurya D. Health Benefits of Quercetin in Age-Related Diseases. Molecules. 2022;27(8). [Google Scholar]
- Dinda B, Dinda M, Dinda S, Ghosh P, Das S. Anti-SARS-CoV-2, Antioxidant and Immunomodulatory Potential of Dietary Flavonol Quercetin: Focus on Molecular Targets and Clinical Efficacy. Eur J Med Chem Rep. 2024;10. [Google Scholar]
- Tu K, Lie J, Wan C, Cameron M, Austel A, Nguyen J. Osteoporosis: A Review of Treatment Options. P T. 2018;43(2):92-104. [Google Scholar]
- Lin X, Patil S, Gao Y, Qian A. The Bone Extracellular Matrix in Bone Formation and Regeneration. Front Pharmacol. 2020;11. [Google Scholar]
- Kim J, Kim M, Hong S, Kim E, Lee H, Jung H. Albiflorin Promotes Osteoblast Differentiation and Healing of Rat Femoral Fractures Through Enhancing BMP-2/Smad and Wnt/β-Catenin Signaling. Front Pharmacol. 2021;12. [Google Scholar]
- Kim J, Kim M, Hong S, Kim E, Lee H, Jung H. Albiflorin Promotes Osteoblast Differentiation and Healing of Rat Femoral Fractures Through Enhancing BMP-2/Smad and Wnt/β-Catenin Signaling. Front Pharmacol. 2021;12. [Google Scholar]
- Sinha K, Zhou X. Genetic and Molecular Control of Osterix in Skeletal Formation. J Cell Biochem. 2013;114(5):975-84. [Google Scholar]
- Guo C, Yang R, Jang K, Zhou X, Liu Y. Protective Effects of Pretreatment with Quercetin Against Lipopolysaccharide-Induced Apoptosis and the Inhibition of Osteoblast Differentiation via the MAPK and Wnt/β-Catenin Pathways in MC3T3-E1 Cells. Cell Physiol Biochem. 2017;43(4):1547-61. [Google Scholar]
- Ahn V, Chu M, Choi H, Tran D, Abo A, Weis W. Structural Basis of Wnt Signaling Inhibition by Dickkopf Binding to LRP5/6. Dev Cell. 2011;21(5):862-73. [Google Scholar]
- Bian W, Xiao S, Yang L, Chen J, Deng S. Quercetin Promotes Bone Marrow Mesenchymal Stem Cell Proliferation and Osteogenic Differentiation through the H19/miR-625-5p Axis to Activate the Wnt/β-Catenin Pathway. BMC Comp Med Ther. 2021;21(1). [Google Scholar]
- Wong R, Rabie A. Effect of Quercetin on Preosteoblasts and Bone Defects. Open Orthop J. 2008;10(2):27-32. [Google Scholar]
- Zhang Y, Yang X, Ge X, Zhang F. Puerarin Attenuates Neurological Deficits via Bcl-2/Bax/Cleaved Caspase-3 and Sirt3/SOD2 Apoptotic Pathways in Subarachnoid Hemorrhage Mice. Biomed Pharmacother. 2019;162:726-33. [Google Scholar]
- Jin C, So Y, Han S, Kim J. Isoegomaketone Upregulates Heme Oxygenase-1 in RAW264.7 Cells via ROS/P38 MAPK/Nrf2 Pathway. Biomol Ther (Seoul). 2016;24(5):510-6. [Google Scholar]
- Biswas P, Dey D, Biswas P, Rahaman T, Saha S, Parvez A. A Comprehensive Analysis and Anti-Cancer Activities of Quercetin in ROS-Mediated Cancer and Cancer Stem Cells. IJMS. 2019;23(19). [Google Scholar]
- Jia H, Zhang Y, Si X, Jin Y, Jiang D, Dai Z. Quercetin Alleviates Oxidative Damage by Activating Nuclear Factor Erythroid 2-Related Factor 2 Signaling in Porcine Enterocytes. Nutrients. 2021;13(2). [Google Scholar]
- Ngo V, Duennwald M. Nrf2 and Oxidative Stress: A General Overview of Mechanisms and Implications in Human Disease. Antioxidants. 2022;11(12). [Google Scholar]
- Zhou Y, Wu Y, Jiang X, Zhang X, Xia L, Lin K. The Effect of Quercetin on the Osteogenesic Differentiation and Angiogenic Factor Expression of Bone Marrow-Derived Mesenchymal Stem Cells. PLoS ONE. 2015;10(6). [Google Scholar]
- Park C, Lee H, Han M, Jeong J, Kim S, Jeong S. Cytoprotective Effects of Fermented Oyster Extracts against Oxidative Stress-Induced DNA Damage and Apoptosis through Activation of the Nrf2/HO-1 Signaling Pathway in MC3T3-E1 Osteoblasts. EXCLI J. 1102;19:1102-9. [Google Scholar]
- Wong S, Chin K, Nirwana S. Quercetin as an Agent for Protecting the Bone: A Review of the Current Evidence. IJMS. 2020;21(17). [Google Scholar]
- Kodama J, Kaito T. Osteoclast Multinucleation: Review of Current Literature. IJMS. 2020;21(16). [Google Scholar]
- Ranger A, Gerstenfeld L, Wang J, Kon T, Bae H, Gravallese E. The Nuclear Factor of Activated T Cells (Nfat) Transcription Factor Nfatp (Nfatc2) Is a Repressor of Chondrogenesis. J Experim Med. 2000;191(1):9-22. [Google Scholar]
- Peng J, Yang Z, Li H, Hao B, Cui D, Shang R. Quercetin Reprograms Immunometabolism of Macrophages via the SIRT1/PGC-1α Signaling Pathway to Ameliorate Lipopolysaccharide-Induced Oxidative Damage. IJMS. 2023;24(6). [Google Scholar]
- Jiang T, Xia T, Qiao F, Wang N, Jiang Y, Xin H. Role and Regulation of Transcription Factors in Osteoclastogenesis. Int J Mol Sci. 2023;24(22). [Google Scholar]
- Forte L, Torricelli P, Boanini E, Gazzano M, Rubini K, Fini M. Antioxidant and Bone Repair Properties of Quercetin-Functionalized Hydroxyapatite: An in Vitro Osteoblast-Osteoclast-Endothelial Cell Co-Culture Study. Acta Biomaterialia. 2016;32:298-308. [Google Scholar]
- Lee K, Seo I, Choi M, Jeong D. Roles of Mitogen-Activated Protein Kinases in Osteoclast Biology. IJMS. 2018;19(10). [Google Scholar]
- Luo M, Tian R, Lu N. Quercetin Inhibited Endothelial Dysfunction and Atherosclerosis in Apolipoprotein E-Deficient Mice: Critical Roles for NADPH Oxidase and Heme Oxygenase-1. J Agric Food Chem. 2020;68(39):10875-83. [Google Scholar]
- Ishikawa Y, Kitamura M. Anti-Apoptotic Effect of Quercetin: Intervention in the JNK- and ERK-Mediated Apoptotic Pathways. Kidney Int. 2000;58(3):1078-87. [Google Scholar]
- Zhou P, Zheng T, Zhao B. Cytokine-Mediated Immunomodulation of Osteoclastogenesis. Bone. 2022;164. [Google Scholar]
- Tang J, Diao P, Shu X, Li L, Xiong L. Quercetin and Quercitrin Attenuates the Inflammatory Response and Oxidative Stress in LPS-Induced RAW264.7 Cells: In Vitro Assessment and a Theoretical Model. BioMed Res Int. 2019;2019:1-8. [Google Scholar]
- Al-Zharani M, Mubarak M, Rudayni H, Al-Doaiss A, Abd-Elwahab M, Al-Eissa M. Quercetin as a Dietary Supplementary Flavonoid Alleviates the Oxidative Stress Induced by Lead Toxicity in Male Wistar Rats. Nutrients. 2023;15(8). [Google Scholar]
- Zhang Y, Zhang Z, Wang R. Protective Mechanisms of Quercetin Against Myocardial Ischemia Reperfusion Injury. Front Physiol. 2020;11. [Google Scholar]
- Jaliah I. Quercetin Inhibits Chronic Stress-Induced Myocardial Infarction in Rats. Int J Morphol. 2017;35(4):1363-9. [Google Scholar]
- Liu H, Guo X, Chu Y, Lu S. Heart Protective Effects and Mechanism of Quercetin Preconditioning on Anti-Myocardial Ischemia Reperfusion (IR) Injuries in Rats. Gene. 2014;545(1):149-55. [Google Scholar]
- Wang Y, Zhang Z, Wu Y, Ke J, He X, Wang Y. Quercetin Postconditioning Attenuates Myocardial Ischemia/Reperfusion Injury in Rats through the PI3K/Akt Pathway. Braz J Med Biol Res. 2013;46(10):861-7. [Google Scholar]
- Liu Y, Song Y, Li S, Mo L. Cardioprotective Effect of Quercetin against Ischemia/Reperfusion Injury Is Mediated Through NO System and Mitochondrial K-ATP Channels. Cell J. 2021;23(2):184-90. [Google Scholar]
- Elbarbry F, Abdelkawy K, Moshirian N, Am AM. The Antihypertensive Effect of Quercetin in Young Spontaneously Hypertensive Rats; Role of Arachidonic Acid Metabolism. Int J Mol Sci. 2020;21(18). [Google Scholar]
- Marino A, Battaglini M, Moles N, Ciofani G. Natural Antioxidant Compounds as Potential Pharmaceutical Tools against Neurodegenerative Diseases. ACS Omega. 2022;7(30):25974-90. [Google Scholar]
- Larson A, Symons J, Jalili T. Therapeutic Potential of Quercetin to Decrease Blood Pressure: Review of Efficacy and Mechanisms. Adv Nutr. 2012;3(1):39-46. [Google Scholar]
- Wang D, Ali F, Liu H, Cheng Y, Wu M, Saleem M. Quercetin Inhibits Angiotensin II-Induced Vascular Smooth Muscle Cell Proliferation and Activation of JAK2/STAT3 Pathway: A Target Based Networking Pharmacology Approach. Front Pharmacol. 2022;13. [Google Scholar]
- Youl E, Bardy G, Magous R, Cros G, Sejalon F, Virsolvy A. Quercetin Potentiates Insulin Secretion and Protects INS-1 Pancreatic Β-cells against Oxidative Damage via the ERK1/2 Pathway. Brit J Pharmacol. 2010;161(4):799-814. [Google Scholar]
- Jin T, Zhang Y, Botchway B, Huang M, Lu Q, Liu X. Quercetin Activates the Sestrin2/AMPK/SIRT1 Axis to Improve Amyotrophic Lateral Sclerosis. Biomed Pharmacother. 2023;161. [Google Scholar]
- Zhang W, Zheng Y, Yan F, Dong M, Ren Y. Research Progress of Quercetin in Cardiovascular Disease. Front Cardiovasc Med. 2023;10. [Google Scholar]
- Dabrowska A, Zajac M, Bednarczyk P, Lukasiak A. Effect of Quercetin on mitoBKCa Channel and Mitochondrial Function in Human Bronchial Epithelial Cells Exposed to Particulate Matter. Int J Mol Sci. 2022;24(1). [Google Scholar]
- Zhou Y, Suo W, Zhang X, Lv J, Liu Z, Liu R. Roles and Mechanisms of Quercetin on Cardiac Arrhythmia: A Review. Biomed Pharmacother. 2022;153. [Google Scholar]
- Scholz E, Zitron E, Katus H, Karle C. Cardiovascular Ion Channels as a Molecular Target of Flavonoids. Cardiovascular Therap. 2010;28(4):46-52. [Google Scholar]
- Medina-Leyte D, García O, Pérez M, Garrido A, Molina T, Albavera L. Endothelial Dysfunction, Inflammation and Coronary Artery Disease: Potential Biomarkers and Promising Therapeutical Approaches. Int J Mol Sci.. 2021;22(8). [Google Scholar]
- Salehi B, Machin L, Monzote L, Rad J, Ezzat S, Salem M. Therapeutic Potential of Quercetin: New Insights and Perspectives for Human Health. ACS Omega. 2020;5(20):11849-72. [Google Scholar]
- Łanoszka K, Vlčková N. Natural Sirtuin1 Activators and Atherosclerosis: An Overview. Curr Atheroscler Rep. 2023;25(12):979-94. [Google Scholar]
- Tian H, Liu Q, Qin S, Zong C, Zhang Y, Yao S. Synthesis and Cardiovascular Protective Effects of Quercetin 7-O-sialic Acid. J Cell Mol Medi. 2017;21(1):107-20. [Google Scholar]
- Cao H, Jia Q, Yan L, Chen C, Xing S, Shen D. Quercetin Suppresses the Progression of Atherosclerosis by Regulating MST1-Mediated Autophagy in Ox-LDL-Induced RAW264.7 Macrophage Foam Cells. IJMS. 2019;20(23). [Google Scholar]
- Li H, Xiao L, He H, Zeng H, Liu J, Jiang C. Quercetin Attenuates Atherosclerotic Inflammation by Inhibiting Galectin-3-NLRP3 Signaling Pathway. Mol Nutr Food Res. 2021;65(15). [Google Scholar]
- Khanamiri F, Berenji M. Quercetin and Heart Health: From Molecular Pathways to Clinical Findings. J Food Biochem. 2023;459095:1-9. [Google Scholar]
- Brociek E, Tymińska A, Giordani A, Ozierański K, Wojnicz R, Grabowski M. Myocarditis: Etiology, Pathogenesis, and Their Implications in Clinical Practice. Biology. 2023;12(6). [Google Scholar]
- Hashemi M, Esmaeeli R, Kahnamouii S, Aghajani S, Frozannia H, Pournasrollah K. Quercetin Decreases Th17 Production by Down-Regulation of MAPK- TLR4 Signaling Pathway on T Cells in Dental Pulpitis. J Dent (Shiraz). 2018;19(4):259-64. [Google Scholar]
- Li Y, Yao J, Han C, Yang J, Chaudhry M, Wang S. Quercetin, Inflammation and Immunity. . Nutrients. 2016;8(3). [Google Scholar]
- Nair M, Mahajan S, Reynolds J, Aalinkeel R, Nair H. The Flavonoid Quercetin Inhibits Proinflammatory Cytokine (Tumor Necrosis Factor Alpha) Gene Expression in Normal Peripheral Blood Mononuclear Cells via Modulation of the NF-Κβ System. Clin Vaccine Immunol. 2006;13(3):319-28. [Google Scholar]
- López N, Grijalva E, Perez D, Heredia J. Flavonoids as Cytokine Modulators: A Possible Therapy for Inflammation-Related Diseases. Int J Mol Sci. 2016;17(6). [Google Scholar]
- Sul O, Ra S. Quercetin Prevents LPS-Induced Oxidative Stress and Inflammation by Modulating NOX2/ROS/NF-kB in Lung Epithelial Cells. Molecules. 2021;26(22). [Google Scholar]
- Nair M, Mahajan S, Reynolds J, Aalinkeel R, Nair H, Schwartz S. The Flavonoid Quercetin Inhibits Proinflammatory Cytokine (Tumor Necrosis Factor Alpha) Gene Expression in Normal Peripheral Blood Mononuclear Cells via Modulation of the NF-Κβ System. Clin Vaccine Immunol. 2006;13(3):319-28. [Google Scholar]
- Cheng S, Huang W, Pang J, Wu Y, Cheng C. Quercetin Inhibits the Production of IL-1β-Induced Inflammatory Cytokines and Chemokines in ARPE-19 Cells via the MAPK and NF-κB Signaling Pathways. Int J Mol Sci. 2019;20(12). [Google Scholar]
- Chekalina N, Burmak Y, Petrov Y, Borisova Z, Manusha Y, Kazakov Y. Quercetin Reduces the Transcriptional Activity of NF-kB in Stable Coronary Artery Disease. Indian Heart J. 2018;70(5):593-7. [Google Scholar]
- Chekalina N, SS, TT, YB, Petrov YY, Manusha Y. Effect of quercetin on parameters of central hemodynamics and myocardial ischemia in patients with stable coronary heart disease. Wiad Lek. 1960;70(4):707-11. [Google Scholar]
- Carrillo-Martinez E, Flores-Hernández F, Salazar-Montes A, Nario-Chaidez H, Hernández-Ortega L. Quercetin, a Flavonoid with Great Pharmacological Capacity. Molecules. 2024;29(5). [Google Scholar]
- Chekalina N, Kazakov Y, Mamontova T, Vesnina L, Kaidashev I. Resveratrol More Effectively than Quercetin Reduces Endothelium Degeneration and Level of Necrosis Factor α in Patients with Coronary Artery Disease. Wiad Lek. 2016;69(3):475-9. [Google Scholar]
- Dave S, Kaur N, Nanduri R, Dkhar H, Kumar A, Gupta P. Inhibition of Adipogenesis and Induction of Apoptosis and Lipolysis by Stem Bromelain in 3T3-L1 Adipocytes. PLoS ONE. 2012;7(1). [Google Scholar]
- Yue J, López J. Understanding MAPK Signaling Pathways in Apoptosis. Int J Mol Sci. 2020;21(7). [Google Scholar]
- Su L, Zeng Y, Li G, Chen J, Chen X. Quercetin Improves High-fat Diet-induced Obesity by Modulating Gut Microbiota and Metabolites in C57BL/6J Mice. Phytother Res. 2022;36(12):4558-72. [Google Scholar]
- Jung C, Cho I, Ahn J, Jeon T, Ha T. Quercetin Reduces High-Fat Diet-Induced Fat Accumulation in the Liver by Regulating Lipid Metabolism Genes. Phytother Res. 2013;27(1):139-43. [Google Scholar]
- Kábelová A, Malínská H, Marková I, Hűttl M, Chylíková B, Šeda O. Quercetin Supplementation Alters Adipose Tissue and Hepatic Transcriptomes and Ameliorates Adiposity, Dyslipidemia, and Glucose Intolerance in Adult Male Rats. Front Nutr. 2022;9. [Google Scholar]
- Chen J. The Cell-Cycle Arrest and Apoptotic Functions of P53 in Tumor Initiation and Progression. Cold Spring Harb Perspect Med. 2016;6(3). [Google Scholar]
- Lotfi N, Yousefi Z, Golabi M, Khalilian P, Ghezelbash B, Montazeri M. The Potential Anti-Cancer Effects of Quercetin on Blood, Prostate and Lung Cancers: An Update. Front Immunol. 2023;14. [Google Scholar]
- Maurya A, Vinayak M. Anticarcinogenic Action of Quercetin by Downregulation of Phosphatidylinositol 3-Kinase (PI3K) and Protein Kinase C (PKC) via Induction of P53 in Hepatocellular Carcinoma (HepG2) Cell Line. Mol Biol Rep. 2015;42(9):1419-29. [Google Scholar]
- Srivastava N, Srivastava R. Curcumin and Quercetin Synergistically Inhibit Cancer Cell Proliferation in Multiple Cancer Cells and Modulate Wnt/β-Catenin Signaling and Apoptotic Pathways in A375 Cells. Phytomedicine. 2019;52:117-28. [Google Scholar]
- Asgharian P, Tazekand A, Hosseini K, Forouhandeh H, Ghasemnejad T, Ranjbar M. Potential Mechanisms of Quercetin in Cancer Prevention: Focus on Cellular and Molecular Targets. Cancer Cell Int. 2022;22(1). [Google Scholar]
- Gupta M, Qin RY. Mechanism and Its Regulation of Tumor-Induced Angiogenesis. World J Gastroenterol. 2003;9(6):1144-55. [Google Scholar]
- Shibuya M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer. 2011;2(12):1097-105. [Google Scholar]
- Baksi R, Singh D, Borse S, Sharma R, Nivsarkar V. Vitro and in Vivo Anticancer Efficacy Potential of Quercetin Loaded Polymeric Nanoparticles. Biomed Pharmacother. 2018;106:1513-26. [Google Scholar]
- Shin E, Lee J, Hong S, Lim T, Byun S. Quercetin Directly Targets JAK2 and PKCδ and Prevents UV-Induced Photoaging in Human Skin. Int J Mol Sci. 2019;20(21). [Google Scholar]
- Mu M, Chakravortty D, Sugiyama T, Koide N, Takahashi K, Mori I. The Inhibitory Action of Quercetin on Lipopolysaccharide-Induced Nitric Oxide Production in RAW 264.7 Macrophage Cells. J Endotoxin Res. 2001;7(6):431-8. [Google Scholar]
- Biancatelli C, Berrill R, Catravas M, Marik J. Quercetin and Vitamin C: An Experimental, Synergistic Therapy for the Prevention and Treatment of SARS-CoV-2 Related Disease (COVID-19). Front Immunol. 2020;11. [Google Scholar]
- Yin Y, Li W, Son Y, Sun L, Lu J, Kim D. Quercitrin Protects Skin from UVB-Induced Oxidative Damage. Toxicol Appl Pharm. 2013;269(2):89-99. [Google Scholar]
- Yang Y, Li Y, Du X, Liu Z, Zhu C, Mao W. Anti-Aging Effects of Quercetin in Cladocera Simocephalus Vetulus Using Proteomics. ACS Omega. 2023;8(20):17609-19. [Google Scholar]
- Eid H, Nachar A, Thong F, Sweeney G, Haddad P. The Molecular Basis of the Antidiabetic Action of Quercetin in Cultured Skeletal Muscle Cells and Hepatocytes. Phcog Mag. 2015;11(41):74-81. [Google Scholar]
- Eid H, Nachar A, Thong F, Sweeney G, Haddad P. The Molecular Basis of the Antidiabetic Action of Quercetin in Cultured Skeletal Muscle Cells and Hepatocytes. Phcog Mag. 2015;11(41):74-81. [Google Scholar]
- Lai P, Zhang L, Ly Y. Quercetin Ameliorates Diabetic Nephropathy by Reducing the Expressions of Transforming Growth Factor-Β1 and Connective Tissue Growth Factor in Streptozotocin-Induced Diabetic Rats. Renal Failure. 2012;34(1):83-7. [Google Scholar]
- Widowati W, Prahastuti S, Tjokropranoto R, Onggowidjaja P, Kusuma H, Afifah E. Quercetin Prevents Chronic Kidney Disease on Mesangial Cells Model by Regulating Inflammation, Oxidative Stress, and TGF-Β1/SMADs Pathway. Peer J. 2022;10. [Google Scholar]
- Ansari P, Choudhury S, Seidel V, Rahman A, Richi A, . Therapeutic Potential of Quercetin in the Management of Type-2 Diabetes Mellitus. Life. 2022;12(8). [Google Scholar]
- Juan C, Lastra PDL, Plou J, Pérez-Lebeña F. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int J Mol Sci. 2021;22(9). [Google Scholar]
- Costa L, Garrick J, Roquè P, Pellacani C. Mechanisms of Neuroprotection by Quercetin: Counteracting Oxidative Stress and More. Oxid Med Cell Long. 2016;2016(1). [Google Scholar]
- Abstract
- Introduction
- Discussion
- Quercetin role in osteoporosis (Op)
- The process through which quercetin facilitates osteoblast-mediated bone growth
- OC-mediated bone resorption inhibition by Quercetin
- Quercetin Role nn Cvs Conditions
- Quercetin Role in Inflammation
- Quercetin Role in Cancer
- Quercetin's Role in Skin Health
- Quercetin Role in Diabetes
- Quercetin's Role in Neurodiverse
- Conclusion
- Conflict of Interest
- Source of Funding
- Acknowledgement
- References
How to Cite This Article
Vancouver
Thombre K, Gupta KR, Sukhdeve S, Sakharwade P, Raut A, Siddiqui A, Umekar M. The natural flavone quercetin: An extensive analysis of its pharmacological mechanisms and medicinal prospects [Internet]. Indian J Pharm Pharmacol. 2024 [cited 2025 Oct 17];11(4):185-194. Available from: https://doi.org/10.18231/j.ijpp.2024.032
APA
Thombre, K., Gupta, K. R., Sukhdeve, S., Sakharwade, P., Raut, A., Siddiqui, A., Umekar, M. (2024). The natural flavone quercetin: An extensive analysis of its pharmacological mechanisms and medicinal prospects. Indian J Pharm Pharmacol, 11(4), 185-194. https://doi.org/10.18231/j.ijpp.2024.032
MLA
Thombre, Kalyani, Gupta, Krishna Radheshyam, Sukhdeve, Sudhanshu, Sakharwade, Pavan, Raut, Aparna, Siddiqui, Amaanullah, Umekar, Mimind. "The natural flavone quercetin: An extensive analysis of its pharmacological mechanisms and medicinal prospects." Indian J Pharm Pharmacol, vol. 11, no. 4, 2024, pp. 185-194. https://doi.org/10.18231/j.ijpp.2024.032
Chicago
Thombre, K., Gupta, K. R., Sukhdeve, S., Sakharwade, P., Raut, A., Siddiqui, A., Umekar, M.. "The natural flavone quercetin: An extensive analysis of its pharmacological mechanisms and medicinal prospects." Indian J Pharm Pharmacol 11, no. 4 (2024): 185-194. https://doi.org/10.18231/j.ijpp.2024.032
